What is Brazing
Brazing is the operation of joining 2 pieces of metal that incorporates employing of heat and addition of filler metal. Filler metal, which features a lower melting point than the metals to be joined, is typically pre-placed or fed into the joint while the parts are heated.In parts with small clearances, the filler is capable of flowing into the joint by capillary action. The temperature of the molten filler utilized for brazing oversteps 800° F.
Brazing can be performed on most metals, and the range of available alloys is rising while new alloys and new service requirements are introduced.
Heating by the torch in the air is adequate if the joint is appropriately fluxed.
Other forms of brazing include electrical resistance, heating, molten salts, and baths of molten metal. The broad use of these forms has led to the development of special furnaces and automatic equipment, with special attention being given to precise control of the temperature and regulation of the atmosphere.
Brazing Methods
The heat for brazing may be exploited by several techniques. The choosing of appropriate brazing methods depends on the shape and size of the joined elements, type of base metal, brazing filler metal, and the rate of production needed. The following brazing methods are typically used in industry:Torch Brazing
It is a heating source catered via a fuel gas flame. Gasses include propane, hydrogen, or acetylene. A most common application is to braze a tube into a fitting applying silver or copper brazing filler metals.Induction Brazing
Electric coils, which are engineered for particular joint geometries, are employed to heat the part and the brazing filler metal as long as the liquid metal flows via capillary action into the joint. This method is primarily used for brazing with silver and copper configuration.Resistance Brazing
If the construction to be brazed are part of the electric circuit, and brazing-heating is produced by resistance to the flow of used current, then this brazing operation is resistance brazing. In a number of applications, the heat is produced by the electrodes. And flows into the metal part, if possible, uniform and straightforward, allowing brazing. Elements should be free to flow unconstrained, even if they expand during heating.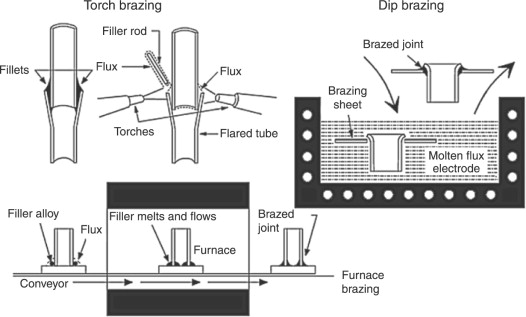
Dip Brazing
This type of brazing consists of applicating brazing-heating through dipping the solid parts either in molten metal or in molten flux, in a bath kept at proper temperature.Bach Furnace
This furnace can be refractory lined and heated by electricity, oil, or gas. Atmospheres can be a generated gas (endothermic or exothermic) or an inert gas such as nitrogen or argon. Gold, nickel, silver, and copper-based brazing filler metals can be brazed successfully in this type of furnaces.Vacuum Furnace
It is a furnace with electrically heated components that encompass the workload and heat the brazing filler metal to the liquids state, so flow and capillary action are achieved. To allow brazing of alloys that are sensitive to oxidation at high temperatures, a pump system is used that removes oxygen. Copper, gold, nickel, titanium, cobalt, and ceramic-based filler metals are successfully vacuum brazed.Infrared Brazing
Brazing-heating can be performed by quartz lamps that release invisible infrared radiation at high power degrees. Reflectors may be utilized to direct the radiation on the parts.Braze Filler Metals
Brazing has 2 important requirements: a filler metal and source of heat to melt the filler. A brazing filler starts with one of several metals such as nickel, cobalt, copper, gold, aluminum, or silver. These base metals are then combined or alloyed, with other metals to improve or tweak their properties. Of course, you must balance a number of options when choosing a filler metal. Alloys behave differently than base metals. Alloys don’t turn directly from a solid to a liquid. Instead, they pass through a semiliquid phase during which they’re both liquid and solid simultaneously. You can use this to your advantage. If you’re brazing a joint with a wider clearance, you may choose a filler with a larger brazing range, which results in a semiliquid flow that can adequately fill the gap. In case, you’re brazing a joint with a narrow clearance, you can choose a filler with a small brazing range, which results in an “active” flow of filler metal in the gap.Braze Filler Metals Forms
Braze filler metals are available in a variety of forms. Where available, you can pick the form that is most suitable and efficient for their particular production needs. All of this depends on how the filler will be applicated. In some cases, you may place the filler in the joint before heating. Also, you can manually feed the filler during the heating process.Below is a list of most used braze filler metal forms:
- Powder: The filler uses the form of dry, spherical fragments. Each fragment has the elements making up the alloy in the right proportion;
- Paste: A paste mixes alloy powders with a neutral binder to produce a filler that can be forced into a joint. Binders can be water or an organic compound and can impact on the brazed joints drying time;
- Tape: Manufacturers exploit binders to attach thin ribbons of brazing metal to an adhesive backing, which can be shipped and rolled up. They often cut the tape thus its dimensions (width and thickness) comply with a metalworker's accurate specifications;
- Foil: Braze foil is a thin and pure sheet of metal alloy. Foil can be cut into pieces and shapes and assembled to create filler of different thickness;
- Rod: Brazing rods include diameters ranging from 0.3125 to 0.375 inches. Like foil, rods have just the elements that making up the alloy, with no binders added.
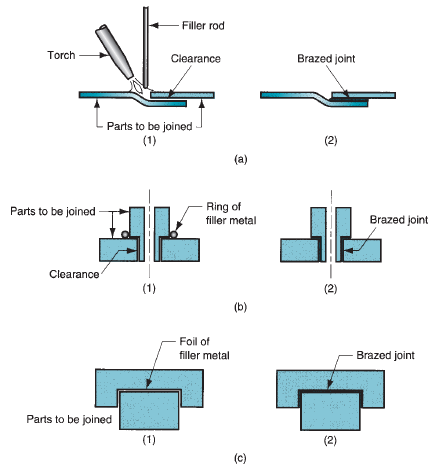
Brazing Flux
The fluxes employed in brazing include a combination of borax, boric acid, borates, fluorides, chlorides along with a wetting agent. The flux may be in the form of a liquid, slurry, powder, or paste, depending upon the brazing method used. A typical composition is 75% borax and 25% boric acid. For brazing of, aluminum, copper alloys, or stainless steel alkaline bi-fluorides are utilized as a flux. A special flux with sodium cyanide is applicated for brazing tungsten to copper. The technique of using the flux may be a pressurized injector, spraying, or a brushing.Braze Joint
There are only 2 basic braze joint types, the lap joint, and butt joint. The braze process chosen and the base metals applied will inform the specific clearance and configuration of the joint. All joint designs should use adequate clearances, exact machining details to avoid the formation of flux traps within the joint, the flow of flux out of the joint, and provision for venting of brazing gasses. A joint design which looks that the brazing filler metal has thoroughly flowed through the joint is recommended.How Strong is Brazing?
Brazing should be considered whenever you need permanent, strong joints. Still, the decision depends on many aspects, like the joint configuration, size of the parts to be joined, the number of joints to be made, and the thickness of the elements. Brazing is a lot stronger than soldering, and unlike many other production processes, it is perfect for joining different materials. Brazing is a flexible technique that fabricates a durable, leak-proof, and permanent joint. This joining method is utilized to produce a wide variety of applications like automotive parts, air conditioners, aerospace components, lightbulbs, refrigeration systems, industrial valves, jet engines, turbines, gas distribution systems, and other objects exposed to various temperature extremes.Brazing Benefits
- Components can be batch processed
- Brazing is cost and production efficient
- Component distortion is reduced or eliminated
- Base metal dilution is low
- Process thermal cycles are anticipated
- Joining of different materials can be accomplished
- Thin-to-Thick or Thin-to-Thin metals can be joined
- Full and small gap sizes can be filled efficiently
- Specialized labor isn’t needed.
What is the Difference Between Brazing and Soldering?
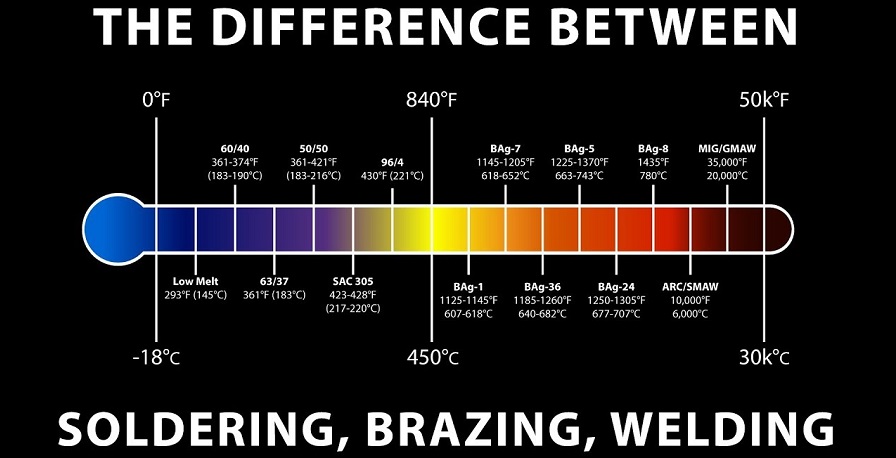
Soldering and brazing both are metal joining methods employed in different joining conditions. These methods are sometimes difficult to differentiate since both techniques apply filler material and run below the critical temperature.
The chief difference between soldering and brazing is that soldering is utilized to produce an electrically strong joint between metals which can withstand all electric loads. While brazing is used to create a mechanically strong joint, which can withstand all mechanic stresses and loads.
Metals that may be soldered include gold, silver, copper, brass, and iron. If the filler metal melts below 840° F, the process being performed is soldering.
Metallurgically, the higher temperatures required for brazing results in very different joint properties in service as compared to soldering. Since the lower temperatures of soldering usually allow only minimal inter-alloying of the filler metal with the pure metals being joined, brazing often outcomes in extensive interaction between the base metal and filler metal.
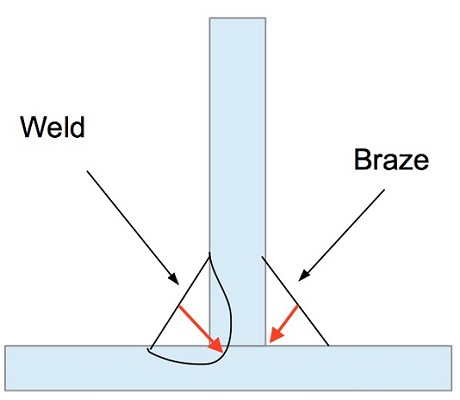
What is the Difference Between Brazing and Welding?
Welding and brazing are two ways of joining two or more pieces of metal. The application, design, and the material of your product determine which process you prefer. The main difference is in temperature since brazing doesn’t melt the base metals. It means that brazing temperatures are permanently lower than the melting points of the base metals.Brazing temperatures are also much lower than welding temperatures for the same base metals, applying less energy.
Since the heat applicated in brazing is less intense, this process doesn’t change most physical properties while minimizes warping, stresses, and distortion, in the joint sector. Additionally, brazing’s lower temperatures waste less energy on the projects.
Brazing is a better option than welding when joining different metals. As long as the filler material is metallurgically compatible with both base metals and melts a lower temperature, brazing can create strong joints without any change of the base metal’s properties.
In welding base metals are melted so joining two different metals using this method can require complicated and costly processes.